

Many of the 700,000 fossils in the Museum’s collections are also made of calcium minerals. The museum’s collections holds nearly 750,000 marine invertebrates, including crustaceans, corals and bristleworms. They are found on either side of the stomach and provide calcium for essential parts of the cuticle such as mouthparts and legs. Calcium required after moulting in lobsters, crawfish, crayfish and some land crabs is provided by gastroliths (sometimes referred to as gizzard stones, stomach stones or crab’s eyes).
/calcium-chemical-element-186451009-5810f6d25f9b58564c68f22a.jpg)
Crustaceans such as crabs and lobsters have a hard exoskeleton strengthened with both calcium carbonate and calcium phosphate. Coral reefs and some marine bristle worm tubes (Serpulidae, Spirorbinae) rely on the reinforcing nature of calcium carbonate to provide support and protection to their soft bodies. Mollusc shells are created as protective shields by their soft-bodied owners and this is true of other invertebrates, especially in the world’s oceans. In the pearl industry the oyster or mussel is ‘seeded’ with a tiny orbs of shell to ensure that the resulted pearl is totally spherical. The mantle around the soft bodied animal secretes calcium carbonate and conchiolin that surrounds the invading body and imitates its shape so they are not all perfectly spherical.

In nature pearls are the result of the molluscs’ reaction against a parasitic intruder or a piece of grit. Pearls, also made of aragonite and calcite, are produced by bivalves such as oysters, freshwater mussels and even giant clams. An international collection it contains many rare, beautiful and scientifically important specimens and is utilised by worldwide scientists for their research. The 5 th most common element in the earth’s crust, calcium forms many useful rocks and minerals such as limestone, aragonite, gypsum, dolomite, marble and chalk.Īragonite and Calcite, the two most commonly crystalised forms of calcium carbonate, helped form the 2 million shells in our mollusc collection, the core of which is the Melvill-Tomlin collection, donated to the museum in the 1950s. It is crucial for life today and commonly forms a supporting role in plants and animals. Known by most as the fundamental element in bone-forming or limestone, it has a host of other applications and is present in seabeds and marine life past and present.Ĭalcium (Ca) is a light-coloured metallic element with an atomic number of 20. One of the most famous compounds is limestone (CaCO 3).Continuing the international year of the periodic table of chemical elements, for April we have selected Calcium. In nature, calcium is always found in compounds with other elements. You won't find calcium sitting around as a pure element. It shouldn't surprise you that calcium has a valence of 2. Those elements in the second column have two electrons ready to make compounds. They all have an outer shell with two electrons and are very reactive. Yes, calcium is defined as a metal because of both its physical and chemical traits. Those elements make up the alkali earth metal family. You will find calcium in the second column of the period table with other elements including beryllium and magnesium. So remember when you look at our breakdown that the electrons aren't always in a nice neat order as shown here. They are found in clouds that can have different shapes that include spheres and dumbbell-like shapes. As you learn more about atomic structure, you will learn that the electrons don't stay in defined areas around the nucleus. The electrons like to be in separate shells/orbitals. In an atom, the electrons spin around the center, also called the nucleus. Each of those colored balls is an electron. If you think this is a little over your head, go back and look at the elements 1-18 that have organizations that are a little more simple. Let's take a look at the arrangements of electrons in the basic elements (left and right sides of the table) of period four and the more complex arrangements of the transition elements (in the middle of the row).
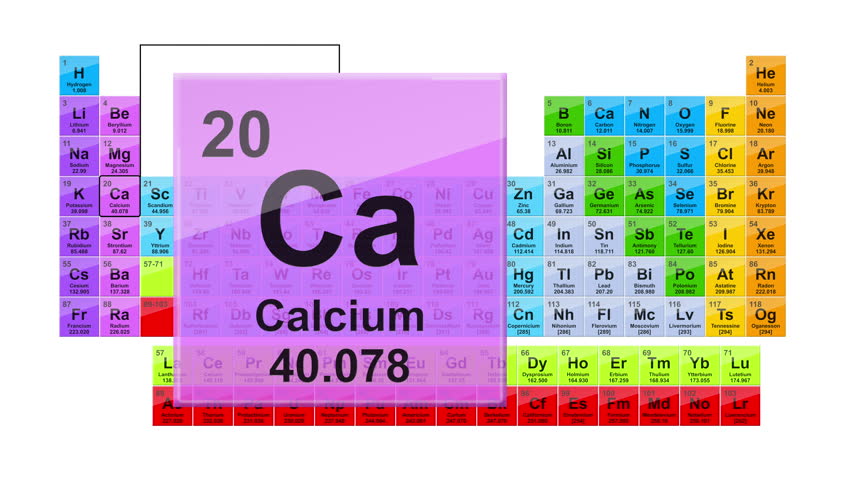
You may have an easy way to know the number of electrons in a neutral atom, but the placement of those electrons gets a little more complex. Now we're working with the fourth period/row in the table of elements. It tells you the mass of one atom, how many pieces are inside, and where it should be placed on the periodic table. That box on the left has all of the information you need to know about one element.


 0 kommentar(er)
0 kommentar(er)
